Q.
a) Structural steel frames are commonly used for tall and wide buildings. Using diagrams explain a typical steel portal frame details. (10 marks)
b) Determine three (3) methods that commonly be used to protect steel from corrosion. (15 marks)
(25 marks, 2017 Q2)
A.
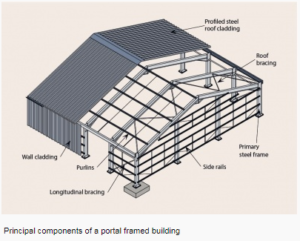
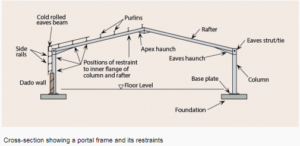
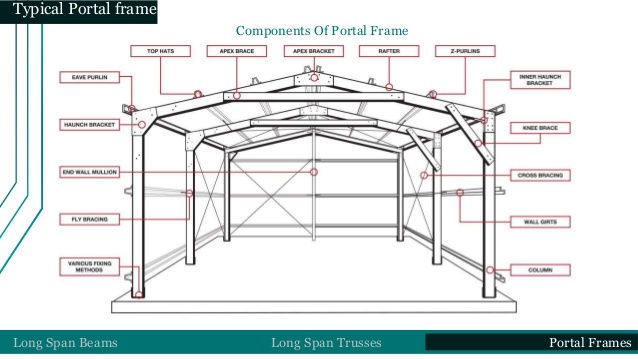
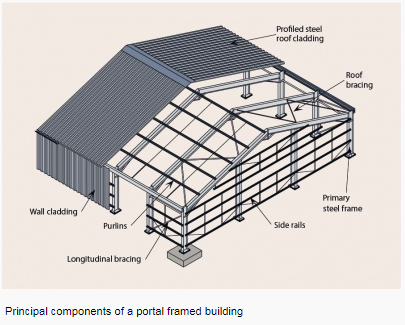
a) Steel portal frame.
Good reading can go here.
Portal frames are generally low-rise structures, comprising columns and horizontal or pitched rafters, connected by moment-resisting connections. Resistance to lateral and vertical actions is provided by the rigidity of the connections and the bending stiffness of the members, which is increased by a suitable haunch or deepening of the rafter sections. This form of continuous frame structure is stable in its plane and provides a clear span that is unobstructed by bracing. Portal frames are very common, in fact 50% of constructional steel used in the UK is in portal frame construction. They are very efficient for enclosing large volumes, therefore they are often used for industrial, storage, retail and commercial applications as well as for agricultural purposes.
Ref:
https://www.slideshare.net/rithikarockingravishankar/long-span-structures-in-concrete-and-steel
b) Methods to prevent corrosion.
Corrosion prevention can take a number of forms depending on the circumstances of the metal being corroded.
- Environmental Modifications
- Metal Selection and Surface Conditions
- Cathodic Protection
- Corrosion Inhibitors
- Coating
- Plating
Environmental Modification
Corrosion is caused by chemical interactions between metal and gasses in the surrounding environment. By removing the metal from, or changing, the type of environment, metal deterioration can be immediately reduced.
This may be as simple as limiting contact with rain or seawater by storing metal materials indoors or could be in the form of direct manipulation of the environmental affecting the metal.
Methods to reduce the sulfur, chloride or oxygen content in the surrounding environment can limit the speed of metal corrosion.
For example, feed water for water boilers can be treated with softeners or other chemical media to adjust the hardness, alkalinity or oxygen content in order to reduce corrosion on the interior of the unit.
No metal is immune to corrosion in all environments, but through monitoring and understanding the environmental conditions that are the cause of corrosion, changes to the type of metal being used can also lead to significant reductions in corrosion.
Metal corrosion resistance data can be used in combination with information on the environmental conditions to make decisions regarding the suitability of each metal.
The development of new alloys, designed to protect against corrosion in specific environments are constantly under production. Hastelloy® nickel alloys, Nirosta® steels, and Timetal® titanium alloys are all examples of alloys designed for corrosion prevention.
Monitoring of surface conditions is also critical in protecting against metal deterioration from corrosion. Cracks, crevices or asperous surfaces, whether a result of operational requirements, wear and tear, or manufacturing flaws, all can result in greater rates of corrosion.
Proper monitoring and the elimination of unnecessarily vulnerable surface conditions, along with taking steps to ensure that systems are designed to avoid reactive metal combinations and that corrosive agents are not used in the cleaning or maintenance of metal parts are all also part of effective corrosion reduction program.
Galvanic corrosion occurs when two different metals are situated together in a corrosive electrolyte.
This a common problem for metals submerged together in seawater, but can also occur when two dissimilar metals are immersed in close proximity in moist soils. For these reasons, galvanic corrosion often attacks ship hulls, offshore rigs, and oil and gas pipelines.
Cathodic protection works by converting unwanted anodic (active) sites on a metal's surface to cathodic (passive) sites through the application of an opposing current. This opposing current supplies free electrons and forces local anodes to be polarized to the potential of the local cathodes.
Cathodic protection can take two forms. The first is the introduction of galvanic anodes.
This method, known as a sacrificial system, uses metal anodes, introduced to the electrolytic environment, to sacrifice themselves (corrode) in order to protect the cathode.
While the metal needing protection can vary, sacrificial anodes are generally made of zinc, aluminum, or magnesium, metals that have the most negative electro-potential.
The galvanic series provides a comparison of the different electro-potential - or nobility - of metals and alloys.
In a sacrificial system, metallic ions move from the anode to the cathode, which leads the anode to corrode more quickly than it otherwise would. As a result, the anode must regularly be replaced.
A second method of cathodic protection is referred to as impressed current protection.
This method, which is often used to protect buried pipelines and ship hulls, requires an alternative source of direct electrical current to be supplied to the electrolyte.
The negative terminal of the current source is connected to the metal, while the positive terminal is attached to an auxiliary anode, which is added to complete the electrical circuit.
Unlike a galvanic (sacrificial) anode system, in an impressed current protection system, the auxiliary anode is not sacrificed.
Corrosion inhibitors are chemicals that react with the metal's surface or the environmental gasses causing corrosion, thereby, interrupting the chemical reaction that causes corrosion.
Inhibitors can work by adsorbing themselves on the metal's surface and forming a protective film. These chemicals can be applied as a solution or as a protective coating via dispersion techniques.
The inhibitors process of slowing corrosion depends upon:
- Changing the anodic or cathodic polarization behavior
- Decreasing the diffusion of ions to the metal's surface
- Increasing the electrical resistance of the metal's surface
Major end-use industries for corrosion inhibitors are petroleum refining, oil and gas exploration, chemical production and water treatment facilities.
The benefit of corrosion inhibitors is that they can be applied in-situ to metals as a corrective action to counter unexpected corrosion.
Paints and other organic coatings are used to protect metals from the degradative effect of environmental gasses.
Coatings are grouped by the type of polymer employed. Common organic coatings include:
- Alkyd and epoxy ester coatings that, when air dried, promote cross-link oxidation
- Two-part urethane coatings
- Both acrylic and epoxy polymer radiation curable coatings
- Vinyl, acrylic or styrene polymer combination latex coatings
- Water-soluble coatings
- High-solid coatings
- Powder coatings
- Plating
Metallic coatings, or plating, can be applied to inhibit corrosion as well as provide aesthetic, decorative finishes.
There are four common types of metallic coatings:
- Electroplating: A thin layer of metal - often nickel, tin, or chromium - is deposited on the substrate metal (generally steel) in an electrolytic bath. The electrolyte usually consists of a water solution containing salts of the metal to be deposited.
- Mechanical plating: Metal powder can be cold welded to a substrate metal by tumbling the part, along with the powder and glass beads, in a treated aqueous solution. Mechanical plating is often used to apply zinc or cadmium to small metal parts
- Electroless: A coating metal, such as cobalt or nickel, is deposited on the substrate metal using a chemical reaction in this non-electric plating method.
- Hot dipping: When immersed in a molten bath of the protective, coating metal a thin layer adheres to the substrate metal.
Sources
Corrosionist.com. Corrosion Control Methods.
Source: www.corrosionist.com
A Guide to Corrosion Protection. Auto/Steel Partnership. 1999.
Source: http://www.a-sp.org/database/custom/cprotection/corrosionprotection.pdf
https://www.thebalance.com/corrosion-prevention-2340000